Include all the lone pairs. We can refer to the periodic table for this.
Which of the compounds exhibit at least one bond angle that is approximately 120 degrees.
. PCl 3 Phosphorus Trichloride Lewis Structure. Use the orbital geometry to predict the hybridization. Draw the Lewis structure for PCl6- in the window below and then answer the questions that follow.
For example if we want to obtain the Lewis structure of the Sulfate ion SO4 2 we must first enter the charge by typing -2 or by entering -2 in the charge field and pressing the Add button. Using Figure contrast the annual rate of oil extraction of the United Using Figure contrast the annual rate of oil extraction of the United States and of Saudi Arabia in 2002 and 2008. It is a positive ion Free radicals are uncharged.
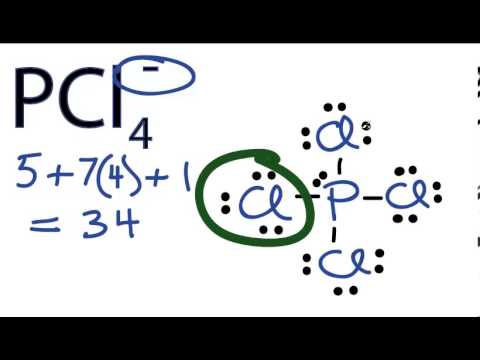
A step-by-step explanation of how to draw the PCl4 Lewis Dot StructureFor the PCl4 structure use the periodic table to find the total number of valence el. Draw the Lewis structure of SO24. Enter the formula of the molecule in the field provided for it.
Find the of lone pairs. Explain why one countrys output increased. Four pairs will be used in the chemical bonds between the P and F.
Which of the compounds have a square planar molecular structure. Draw the Lewis structure. Phosphorus trichloride PCl 3 contains three chlorine atoms and one phosphorus atoms.
S O 2 45. 019158How to Draw the Lewis Structure for PCl4 - - YouTubeYouTube. Draw the Lewis structures for TeCl 4 ICl 5 PCl 5 KrCl 4 and XeCl 2.
It has 4 bonding pairs and no lone pairs. These instructions outline the Kelter strategy to draw Lewis structures for molecules. In the Lewis structure of BF4- there are a total of 32.
Drawing the Lewis Structure for ICl 4-For the ICl4- Lewis structure the total number of valence electrons found on the periodic table for the ICl4- molecule. Ethanol C 2 H 5 OH is used extensively as motor fuel in Brazil. Once we know how many valence electrons there are in ICl4- we can distribute them around the central atom with the goal of filling the outer shells of each atom.
Once we know how many valence electrons there are in BF4- we can distribute them around the central atom with the goal of filling the outer shells of each atom. The Lewis structure of a compound does not deal with the 3-dimensional representation of its elements in space nor its molecular design and geometry. Valence e- 5 64 3 32 e- 16 pairs of bonding pairs Draw single bonds between terminal atoms central atom.
A step-by-step explanation of how to draw the PCl4- Lewis Dot StructureFor the PCl4- structure use the periodic table to find the total number of valence el. Question 5 Covalent bonds form when phosphorus reacts with chlorine to form PCl 3. To use the Lewis Structure Calculator follow these steps.
Also there are no charges on atoms in PCl 5 lewis structure. Drawing the Lewis Structure for BH 4-For the BF4- Lewis structure the total number of valence electrons found on the periodic table for the BF4- molecule. Draw the Lewis resonance structures for the following molecules43.
Drawing the Lewis structure of PCl5. Pairs to each oxygen terminal atoms. Include all the lone pairs.
Now we are going to study each step of drawing the lewis structure of PCl 5 in next sections. Pairs available lone pairs used. Each chlorine at no.
Question 4 The Lewis electron dot structure of the ethanedioate ion is shown below. Electronegativity of A and B atoms should be equal or slightly different from. In the Lewis structure for PCl 4-there are a total of 34 valence electrons.
Draw the Lewis structure for the BeCl42- ion. Use VSEPR Theory to predict the orbital geometry. Do not include overall ion charges or formal charges in your drawing.
Phosphorus uses sp³ orbitals in PCl₄. Find the Total Number of Valence Electrons In this step add up the total number of valence electrons from all the atoms in the molecule. Also there is a lone pair on phosphorus atom.
Lewis structure represents the valence shell electrons in a. There are guidelines to draw a lewis structure properly and easily. What is the shape of an ammonium nh4 ion.
Count the number of valence electrons in a PCl5 molecule. Use section 10 of the data booklet. Draw the Lewis structure of PCl3.
Draw The Lewis Structure For The Pcl 4 Ion. PCl 4-is a negative ion an anion so you have to add an extra valence electron. Steps of drawing PCl 5 lewis structure.
Which of the compounds exhibit d 2 sp 3 hybridization. In PCl 3 lewis structure each chlorine atom is joint with center phosphorus atom through a single bond. Predict its geometry and describe the hybridization state of the Be atom.
Orbitals that point toward the corners of a regular tetrahedron are sp³. A and B atoms should have a higher values of ionization potential so that none of them should be able to lose electron s. O P O.
These days I am unfolding before you twelve simple 3D nail artwork designs ideas trends stickers. Outline why all the CO bond lengths in the ethanedioate ion are the same length and suggest a value for them. Draw the lewis structure for the pcl 4 ion New and most current styles are being launched by specialists so Progressively more women can Stick to the streak of nail artwork.
Tetrahedral The ammonium ion has exactly the same shape as methane because it has exactly the same electronic arrangement. Is ch2ci2 polar or nonpolar. DRAWING LEWIS STRUCTURES Draw the Lewis structure of phosphorous trichloride PCl 3 Draw the Lewis structure for the NO ion 42.
Phosphorus at no 15 has 15 protons and 15 electrons so overall it has no charge. 16 pairs 4 pairs used 12 lone pairs. C Se 8 a cyclic molecule with a ring of eight Se atoms Methanol H 3 COH is used as the fuel in some race cars.
Write Lewis structures for the following molecules or ions. Both methanol and ethanol produce CO 2 and H 2 O when they burn. The bonds should point toward the corners of a regular tetrahedron.
Drawing the Lewis Structure for PCl 4-Viewing Notes. Number of steps can be changed according the complexity of the molecule or ion. Draw the Lewis structure of PCl3.
17 has 17 protons and 17 electrons so overall no change If you add up all of the protons in hypothetical PCl 4 15 17 17 17 17 83. In the Lewis structure of ICl4- there are total of 36. Deduce the Lewis.
You need to do some basic housekeeping. In this tutorial we will learn how to draw the lewis structure of PCl 3 with all theories. Draw the Lewis structure of SO24.
N O 2 - 44. Move Clear Erase Undo Redo Increase Scale Decrease Scale Copy Paste Set Label More Labels Attributes More Attributes Single Bond Recessed Bond Protruding Bond Double Bond Other. In the PCl 4-Lewis structure Phosphorus P is the least electronegative so it goes in the center.
This is an AX₄ ion.

How To Draw The Lewis Structure For Pcl4 Youtube

Pcl4 Lewis Structure How To Draw The Lewis Structure For Pcl4 Youtube

Drawing The Lewis Structure For Pcl4 Youtube

Consider The Phosphorus Tetrachloryl Pcl4 Cation What Is The Central Atom Enter Its Chemical Symbol Study Com

Pcl4 Lewis Structure How To Draw The Lewis Structure For Pcl4 Youtube



0 comments
Post a Comment